Giardia lamblia
| Giardia lamblia | |
|---|---|
 |
|
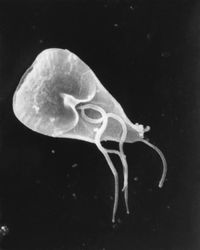
| Giardia cell, SEM | |
| Scientific classification | |
| Domain: | Eukaryota |
| Phylum: | Metamonada |
| Order: | Diplomonadida |
| Family: | Hexamitidae |
| Genus: | Giardia |
| Species: | G. lamblia |
| Binomial name | |
| Giardia intestinalis (Lambl, 1859) Kofoid & Christiansen, 1915 |
|
Giardia lamblia (synonymous with Lamblia intestinalis and Giardia duodenalis) is a flagellated protozoan parasite that colonises and reproduces in the small intestine, causing giardiasis. The giardia parasite attaches to the epithelium by a ventral adhesive disc, and reproduces via binary fission.[1] Giardiasis does not spread via the bloodstream, nor does it spread to other parts of the gastro-intestinal tract, but remains confined to the lumen of the small intestine.[2] Giardia trophozoites absorb their nutrients from the lumen of the small intestine, and are anaerobes. If the organism is split and stained, it has a very characteristic pattern that resembles a familiar "smiley face" symbol.
Contents |
Hosts
Giardia infects humans, but is also one of the most common parasites infecting cats, dogs and birds. Mammalian hosts also include cows, beavers, deer, and sheep.
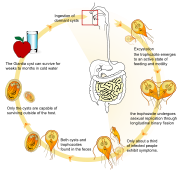
Life cycle
Giardia infection can occur through ingestion of dormant cysts in contaminated water, food, or by the faecal-oral route (through poor hygiene practices). The Giardia cyst can survive for weeks to months in cold water,[3] and therefore can be present in contaminated wells and water systems, especially stagnant water sources such as naturally occurring ponds, storm water storage systems, and even clean-looking mountain streams. They may also occur in city reservoirs and persist after water treatment, as the Giardia cysts are resistant to conventional water treatment methods such as chlorination and ozonolysis.[3] Zoonotic transmission is also possible, and therefore Giardia infection is a concern for people camping in the wilderness or swimming in contaminated streams or lakes, especially the artificial lakes formed by beaver dams (hence the popular name for giardiasis, "Beaver Fever").
In addition to waterborne sources, fecal-oral transmission can also occur, for example in day care centers, where children may have poor hygiene practices. Those who work with children are also at risk of being infected, as are family members of infected individuals. Not all Giardia infections are symptomatic, and many people can unknowingly serve as carriers of the parasite.
The life cycle begins with a noninfective cyst being excreted with the feces of an infected individual. The cyst is hardy, providing protection from various degrees of heat and cold, desiccation, and infection from other organisms. A distinguishing characteristic of the cyst is four nuclei and a retracted cytoplasm. Once ingested by a host, the trophozoite emerges to an active state of feeding and motility. After the feeding stage, the trophozoite undergoes asexual replication through longitudinal binary fission. The resulting trophozoites and cysts then pass through the digestive system in the faeces. While the trophozoites may be found in the faeces, only the cysts are capable of surviving outside of the host.
Distinguishing features of the trophozoites are large karyosomes and lack of peripheral chromatin, giving the two nuclei a halo appearance. Cysts are distinguished by a retracted cytoplasm. This protozoan lacks mitochondria, although the discovery of the presence of mitochodrial remnants organelles in one recent study "indicate that Giardia is not primitively amitochondrial and that it has retained a functional organelle derived from the original mitochondrial endosymbiont"[4] This organelle is now termed a mitosome.
Intracellular Metabolism and biochemistry
Giardia relies on glucose as its major energy source and breaks glucose down into ethanol, acetate and carbon dioxide.[5] However, it can also use arginine as an energy source.[6] Giardia possesses unique biochemical pathways that suggest that it diverged from other eukaryotes at an early stage in evolution.[6]
B vitamins and bile salts are necessary for Giardia to survive, and a low-carbohydrate diet was shown in mice to reduce the number of Giardia organisms present.[7]
Manifestation of infection
Nomenclature for Giardia species is difficult, as humans and other animals appear to have morphologically identical parasites.
Colonization of the gut results in inflammation and villous atrophy, reducing the gut's absorptive capability. In humans, infection is symptomatic only about 50% of the time, and protocol for treating asymptomatic individuals is controversial.[3] Symptoms of infection include (in order of frequency) diarrhea, malaise, excessive gas (often flatulence or a foul or sulphuric-tasting belch, which has been known to be so nauseating in taste that it can cause the infected person to vomit), steatorrhoea (pale, foul smelling, greasy stools), epigastric pain, bloating, nausea, diminished interest in food, possible (but rare) vomiting which is often violent, and weight loss.[3] Pus, mucus and blood are not commonly present in the stool. It usually causes "explosive diarrhea" and while unpleasant, is not fatal. In healthy individuals, the condition is usually self-limiting, although the infection can be prolonged in patients who are immunocompromised, or who have decreased gastric acid secretion.[3]
People with recurring Giardia infections, particularly those with a lack of IgA, may develop chronic disease.
Lactase deficiency may develop in an infection with Giardia, however this usually does not persist for more than a few weeks, and a full recovery is the norm.
Some studies have shown that giardiasis should be considered as a cause of vitamin B12 deficiency, this a result of the problems caused within the intestinal absorption system.[8]
Prevention
Treatment of drinking water for Giardia is ordinarily indicated in wilderness regions in North America,[9][10] although at least four researchers disagree with this statement, including Robert W. Derlet, a professor at the University of California-Davis School of Medicine, Timothy P. Welch and Thomas R. Welsh of Tulane Medical School and the Children's Hospital of Cincinnati respectively, and Robert Rockwell, a widely quoted writer who is an engineer by training.[11][12][13][14]
Treatment and diagnosis
Giardia lamblia infection in humans is frequently misdiagnosed. Accurate diagnosis requires an antigen test or, if that is unavailable, an ova and parasite examination of stool. Multiple stool examinations are recommended, since the cysts and trophozoites are not shed consistently. Given the difficult nature of testing to find the infection, including many false negatives, some patients should be treated on the basis of empirical evidence; treating based on symptoms.[15]
Human infection is conventionally treated with metronidazole, tinidazole or nitazoxanide. Although Metronidazole is the current first-line therapy, it is mutagenic in bacteria and carcinogenic in mice, so should be avoided during pregnancy.[3] It has not directly been linked to causing cancer in humans, only in other mammals, therefore appears safe. One of the most common alternative treatments is berberine sulfate (found in Oregon grape root, goldenseal, yellowroot, and various other plants). Berberine has been shown to have an antimicrobial and an antipyretic effect.[16] Berberine compounds cause uterine stimulation, and so should be avoided in pregnancy. Continuously high dosing of berberine may lead to bradycardia and hypotension in some individuals.[17]
| Drug | Treatment duration | Possible Side Effects |
|---|---|---|
| Metronidazole | 5–7 days | Metallic taste; nausea; vomiting; dizziness; headache; disulfiram-like effect; neutropenia |
| Tinidazole | Single dose | Metallic taste; nausea; vomiting; belching; dizziness; headache; disulfiram-like effect |
| Nitazoxanide | 3 days | Abdominal pain; diarrhea; vomiting; headache; yellow-green discolouration of urine |
| Albendazole | 5 days | Dizziness; headache; fever; nausea; vomiting; temporary hair loss |
Table adapted from Huang, White.[3]
Treatment in animals
Cats can be cured easily, lambs usually simply lose weight, but in calves the parasites can be fatal and often are not responsive to antibiotics or electrolytes. Carriers among calves can also be asymptomatic. Dogs have a high infection rate, as 30% of the population under one year old are known to be infected in kennels. The infection is more prevalent in puppies than in adult dogs. This parasite is deadly for chinchillas, so extra care must be taken by providing them with safe water. Infected dogs can be isolated and treated, or the entire pack at a kennel can be treated together regardless. Kennels should also be then cleaned with bleach or other cleaning disinfectants. The grass areas used for exercise should be considered contaminated for at least one month after dogs show signs of infection, as cysts can survive in the environment for long periods of time. Prevention can be achieved by quarantine of infected dogs for at least 20 days and careful management and maintenance of a clean water supply.
Microscopy
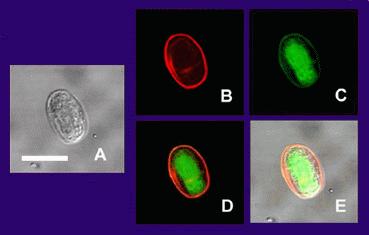
(A) is the cyst imaged by transmission (differential interference contrast), only.
(B) is the cyst wall selectively imaged through use of fluorescent-labelled (TRITC) antibody that is cyst wall specific.
(C) is the cyst imaged through use of carboxy fluorescein diacetate, a viability stain.
(D) is a composite image of (B) and (C).
(E) is a composite image of (A), (B), and (C).
Under a normal compound light microscope, Giardia often looks like a "clown face," with two nuclei outlined by adhesive discs above dark median bodies that form the "mouth." Cysts are oval, have four nuclei, and have clearly visible axostyles. In spite of the common belief that all Eukaryotes have mitochondria, Giardia is one of the few that lack these organelles.
Research
Giardia alternates between two different forms — a hardy, dormant cyst that contaminates water or food and an active, disease-causing form that emerges after the parasite is ingested. National Institute of General Medical Sciences grantee Dr. Frances Gillin of the University of California, San Diego and her colleagues cultivated the entire life cycle of this parasite in the laboratory, and identified biochemical cues in the host's digestive system which trigger Giardia's life cycle transformations.[18][19] They also uncovered several ways in which the parasite evades the defences of the infected organism. One of these is by altering the proteins on its surface, which confounds the ability of the infected animal's immune system to detect and combat the parasite (called antigenic variation). Gillin's work reveals why Giardia infections are extremely persistent and prone to recur. In addition, these insights into Giardias biology and survival techniques may enable scientists to develop better strategies to understand, prevent, and treat Giardia infections.
In December 2008, Nature published an article showing the discovery of an RNA Interference mechanism that allows Giardia to switch VSPs (Variant-Specific Surface Proteins) to avoid host immune response. The discovery was made by the team working at the Biochemistry and Molecular Biology Laboratory, School of Medicine, Catholic University of Cordoba, Argentina, lead by Dr. Hugo Lujan.
Genomics
Giardia and the other diplomonads are unique in their possession of two nuclei that are similar in appearance, DNA content, transcription and time of replication. There are five chromosomes per the haploid genome The genome has been sequenced and was published in 2007, although the sequence contains several gaps. The sequence is approximately 12 million base pairs and contains approximately 5000 protein coding genes.[20] The GC content is 46%. Trophozoites have a ploidy of four and the ploidy of cysts is eight, which in turn raises the question of how Giardia maintains homogeneity between the chromosomes of the same and opposite nuclei. Modern sequencing technologies have been used to resequence different strains.[21]
Giardia has been assumed to be primitively asexual and with no means of transferring DNA between nuclei. These assumptions make it very difficult to explain the remarkably low level of allelic heterozygosity (< 0.01%) in the genome isolate, WB. However, all these assumptions are now in doubt with the identification of meiotic genes, evidence for recombination among isolates and the evidence for exchange of genetic material between nuclei during the process of encystation.[22]
Seven genotypes have been recognised to date (A-G). Of these B is the most widespread. Only types A and B have been shown to be infectious to humans.
History
The trophozoite form of Giardia was first observed in 1681 by Antonie van Leeuwenhoek in his own diarrhea stools. The organism was again observed and described in greater detail by Vilém Dušan Lambl in 1859, who thought the organism belonged to the genus Cercomonas and proposed the name Cercomonas intestinalis. His name is still sometimes attached to the genus or the species infecting humans. Thereafter, some have named the genus after him while others have named the species of the human form after him Giardia lamblia. In 1879, Grassi discovered a rodent parasite – now known to be a Giardia species – Dimorphus muris apparently unaware of Lambl's earlier description. In 1882 and 1883, Johann Künstler described an organism in tadpoles (possibly Giardia agilis) that he named Giardia, this being the first time Giardia was used as a genus name. The genus was chosen to honour Professor Alfred Mathieu Giard of Paris. Raphaël Blanchard, in 1888, proposed the name Lamblia intestinalis,[23] after Lambl. Stiles changed it to Giardia duodenalis in 1902 and to Giardia lamblia in 1915.[24] The same year (1915), Kofoid and Christiansen wrote "The generic name Lamblia Blanchard 1888 sould give way to Giardia Kunstler 1882 on ground of priority…"[25] (the epithet being intestinalis) and used Giardia enterica in 1920.
The naming of the species still causes controversy. While initially species names were based on the host of origin leading to over forty species. In 1922 Simon, using morphologic criteria to distinguish between Giardia lamblia and Giardia muris accepted the name Giardia lamblia for the human species. Filice in 1922 further revised the genus when he published a detailed morphologic description of the genus Giardia and proposed that three species names be used on the basis of the morphology of the median body: Giardia agilis, Giardia duodenalis and Giardia muris.
The names for the human parasite Giardia duodenalis, Giardia lamblia and Giardia intestinalis are all in common current use despite the potential for confusion that this has created.
His observations were recreated, using a single lensed microscope of the kind used by Leeuwenhoek, by British microbiologist Brian J. Ford who showed how clearly one could view Giardia through a primitive microscope.[26]
In 1998, a highly publicised Giardia and Cryptosporidium outbreak was reported in Sydney, Australia, but it was found to be due to mis-measurement of the concentrations of microbes in the water supply. A 2004 outbreak in Bergen (Norway) hastened work on adding UV treatment to the water facilities. In October 2007, Giardia was found in the water supply for parts of Oslo, prompting authorities to advise the public to boil drinking water;[27] but subsequent test showed levels of contamination too low to pose a threat, so this advice has since been cancelled.[28]
In 2008, Giardia was identified as one of the causes of the dysentery afflicting Crusaders in Palestine in the 12th and 13th centuries.[29]
See also
- List of parasites (human)
- 1998 Sydney water crisis
References
- ↑ Oxford textbook of Medicine, Fourth Edition, Volume 1 (2003) Oxford University Press pp.759-760 ISBN 0192629220
- ↑ Harrison's Internal Medicine, Harrison's Online Chapter 199 Protozoal intestinal infections and trochomoniasis
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Huang DB, White AC (2006). "An updated review on Cryptosporidium and Giardia". Gastroenterol. Clin. North Am. 35 (2): 291–314, viii. doi:10.1016/j.gtc.2006.03.006. PMID 16880067.
- ↑ Tovar J, León-Avila G, Sánchez LB, et al. (2003). "Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation". Nature 426 (6963): 172–6. doi:10.1038/nature01945. PMID 14614504.
- ↑ "Giardia - MicrobeWiki". Microbewiki.kenyon.edu. http://microbewiki.kenyon.edu/index.php/Giardia. Retrieved 2010-07-29.
- ↑ 6.0 6.1 Brown DM, Upcroft JA, Edwards MR, Upcroft P (1998). "Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis". International Journal for Parasitology 28 (1): 149–64. doi:10.1016/S0020-7519(97)00172-0. PMID 9504342. http://www.mitochondrial.net/showabstract.php?pmid=9504342.
- ↑ Erlandsen; Meyer (1984). Giardia and Giardiasis. New York: Plenum Press. ISBN 0306415399.
- ↑ Cordingley FT, Crawford GP (1986). "Giardia infection causes vitamin B12 deficiency". Australian and New Zealand Journal of Medicine 16 (1): 78–9. PMID 3458451.
- ↑ Betancourt, WQ; Rose, JB (2004). "Drinking water treatment processes for removal of Cryptosporidium and Giardia.". Veterinary parasitology 126 (1-2): 219–34. doi:10.1016/j.vetpar.2004.09.002. PMID 15567586.
- ↑ Exner, M; Gornik, V (2004). "Parasitic zoonoses transmitted by drinking water. Giardiasis and cryptosporidiosis". Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 47 (7): 698–704. doi:10.1007/s00103-004-0863-y. PMID 15254826.
- ↑ Welch TP (2000). "Risk of giardiasis from consumption of wilderness water in North America: a systematic review of epidemiologic data". International Journal of Infectious Diseases 4 (2): 100–3. doi:10.1016/S1201-9712(00)90102-4. PMID 10737847.
- ↑ Derlet, Robert W. "High Sierra Water: What is in the H20?" Sierra Nature Notes, Volume 3, April 2004.
- ↑ Welch TR (2004). "Evidence-based medicine in the wilderness: the safety of backcountry water". Wilderness & Environmental Medicine 15 (4): 235–7. PMID 15636372. http://www.wemjournal.org/wmsonline/?request=get-document&issn=1080-6032&volume=015&issue=04&page=0235.
- ↑ Wood, T.D. "Water: What Are the Risks?" REI Expert Advice, February 2008.
- ↑ Curtis, Rick. "Outdoor Action Guide to Giardia, Lyme Disease and other 'post trip' Illnesses." Outdoor Action, 2005-2008.
- ↑ Kaneda Y, Tanaka T, Saw T (1990). "Effects of berberine, a plant alkaloid, on the growth of anaerobic protozoa in axenic culture". The Tokai Journal of Experimental and Clinical Medicine 15 (6): 417–23. PMID 2131648.
- ↑ UpToDate (Lexi-Comp, Inc.) retrieved 28 August 2007
- ↑ Hetsko ML, McCaffery JM, Svärd SG, Meng TC, Que X, Gillin FD (1998). "Cellular and transcriptional changes during excystation of Giardia lamblia in vitro". Experimental Parasitology 88 (3): 172–83. doi:10.1006/expr.1998.4246. PMID 9562420.
- ↑ Svärd SG, Meng TC, Hetsko ML, McCaffery JM, Gillin FD (1998). "Differentiation-associated surface antigen variation in the ancient eukaryote Giardia lamblia". Molecular Microbiology 30 (5): 979–89. doi:10.1046/j.1365-2958.1998.01125.x. PMID 9988475.
- ↑ Morrison HG, McArthur AG, Gillin FD, et al. (2007). "Genomic minimalism in the early diverging intestinal parasite Giardia lamblia". Science 317 (5846): 1921–6. doi:10.1126/science.1143837. PMID 17901334.
- ↑ Franzén O, Jerlström-Hultqvist J, Castro E, et al. (2009). "Draft genome sequencing of giardia intestinalis assemblage B isolate GS: is human giardiasis caused by two different species?". PLoS Pathogens 5 (8): e1000560. doi:10.1371/journal.ppat.1000560. PMID 19696920.
- ↑ Adam, RD and Svard, SG (2010). "Giardia: Nuclear and Chromosomal Structure and Replication". Anaerobic Parasitic Protozoa: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-61-5.
- ↑ Blanchard R (1888). "Remarques sur le mégastome intestinal". Bulletin de la Société Zoologique de France 13: 18.
- ↑ Hegner RW (1922). "The systematic relationship of Giardia lamblia Stiles, 1915, from man and Giardia agilis Künstler 1882 from the tadpole". American Journal of Epidemiology 2 (4): 435. http://aje.oxfordjournals.org/cgi/pdf_extract/2/4/435.
- ↑ Kofoid CA, Christiansen EB (1915). "On the Life-History of Giardia". Proceedings of the National Academy of Sciences of the United States of America 1 (11): 547–52. doi:10.1073/pnas.1.11.547. PMID 16576068.
- ↑ Ford BJ (2005). "The discovery of Giardia". The Microscope 53 (4): 148–153. http://www.brianjford.com/Giardia-14-06.pdf.
- ↑ AVJonathan Tisdall . "Oslo water unsafe - Aftenposten - News in English". Aftenposten.no. http://www.aftenposten.no/english/local/article2052645.ece. Retrieved 2010-07-29.
- ↑ "Hovedside - Vann- og avløpsetaten - Oslo kommune". Vann-og-avlopsetaten.oslo.kommune.no. http://www.vann-og-avlopsetaten.oslo.kommune.no/. Retrieved 2010-07-29.
- ↑ Mitchell, Piers D.; Stern, Eliezer; Tepper, Yotam (2008). "Dysentery in the crusader kingdom of Jerusalem: an ELISA analysis of two medieval latrines in the City of Acre (Israel)". Journal of Archaeological Science 35: 1849. doi:10.1016/j.jas.2007.11.017.
External links
- GiardiaDB: The Giardia lamblia genome sequencing project
- Washington State Department of Health fact sheet on Giardia.
- United States Center for Disease Control fact sheet on Giardia
- United States Environmental Protection Agency fact sheet on Giardia in water
- Giardia article at MicrobeWiki
- Video of Giardia Life Cycle
- Giardia and the Sierra Nevada
- http://diarrhea.emedtv.com/giardia-lamblia/giardia-lambia.html
- Prucca CG, Slavin I, Quiroga R, et al. (2008). "Antigenic variation in Giardia lamblia is regulated by RNA interference". Nature 456 (7223): 750–4. doi:10.1038/nature07585. PMID 19079052. Lay summary – The New York Times (15 December 2008).
|
||||||||||||||||||||||||||||||||